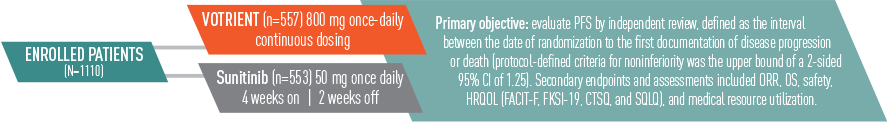
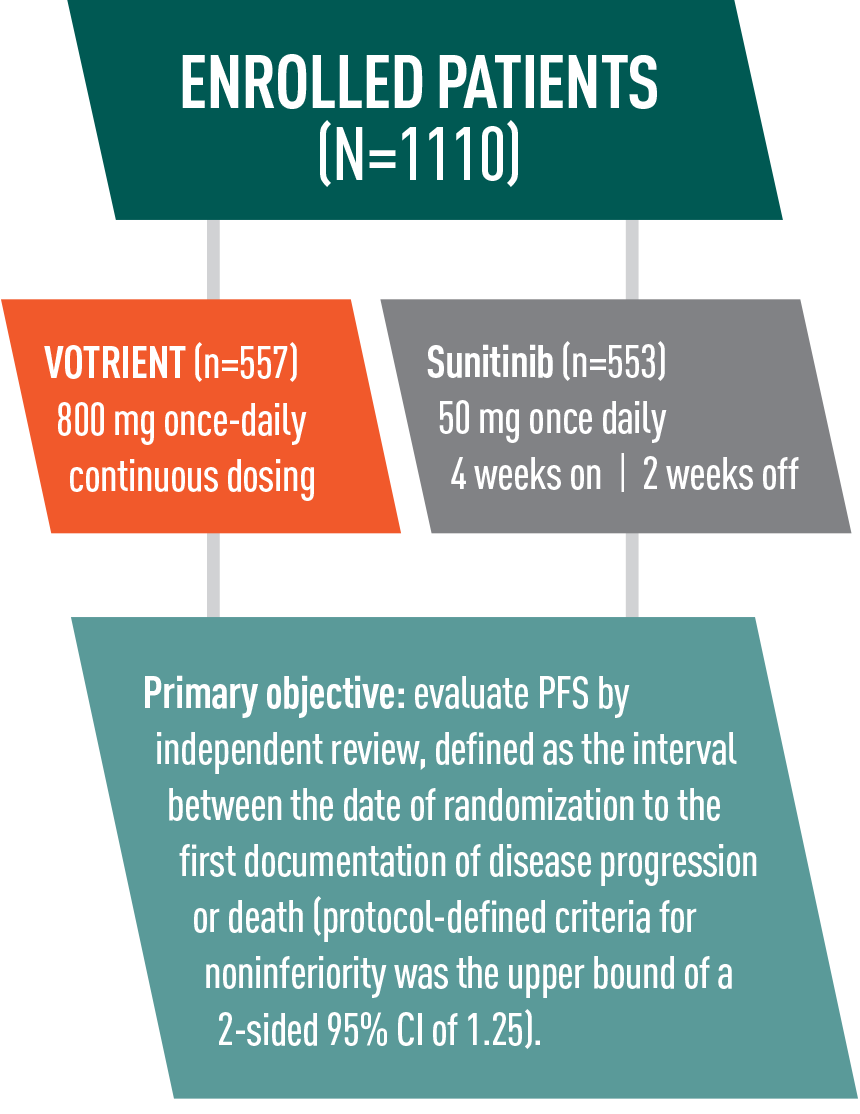
VOTRIENT—The significantly prolonged progression-free survival (PFS) your patients need from the start
9.2 months median PFS for VOTRIENT in the overall treatment population1,2
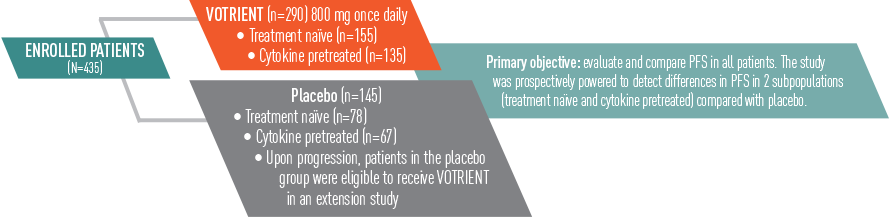
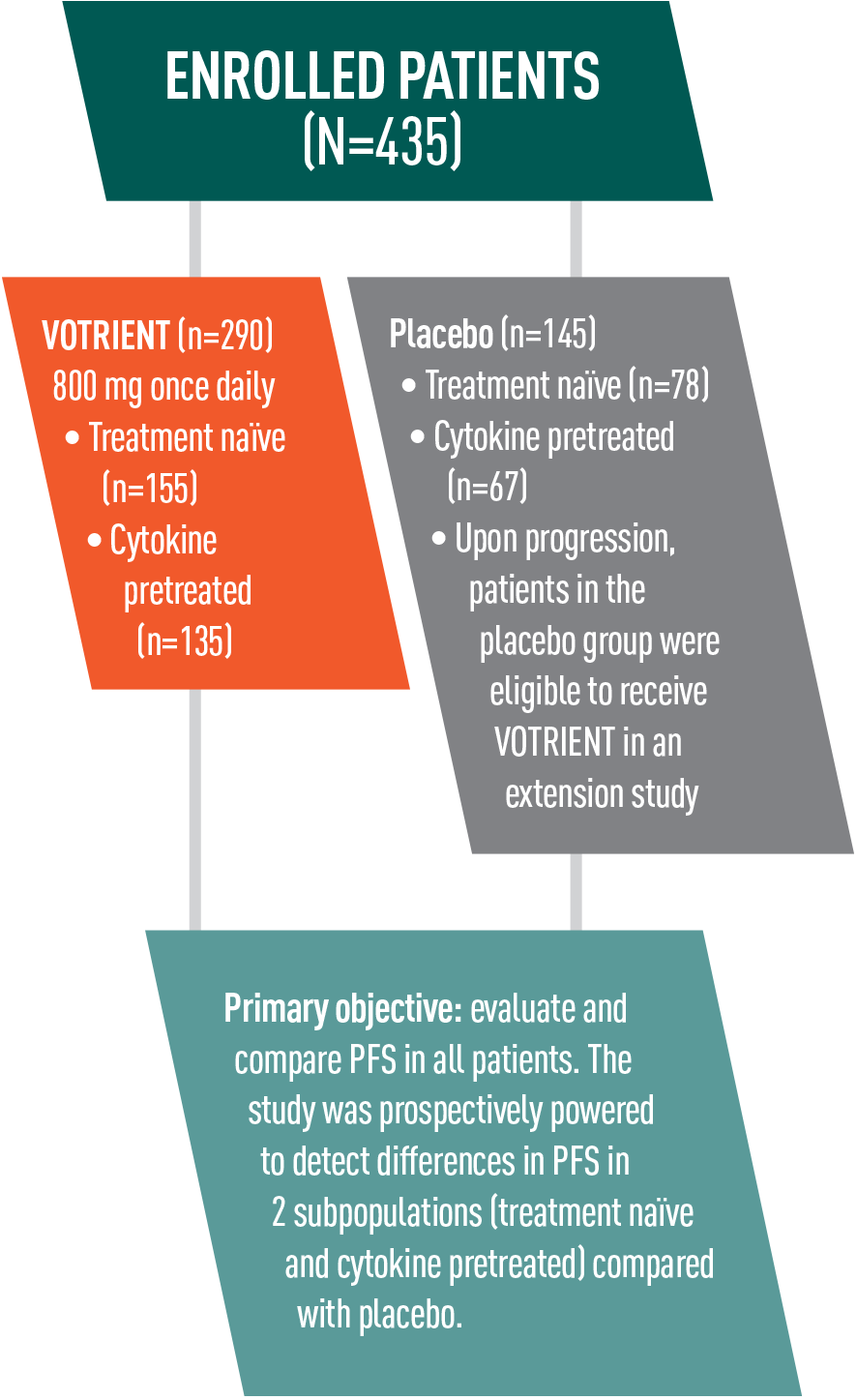
VOTRIENT significantly prolonged PFS vs placebo in the overall treatment population, including treatment-naïve and cytokine pretreated patients1
- VOTRIENT demonstrated a median PFS of 9.2 months vs 4.2 months for placebo (HR=0.46, P<0.0001)*
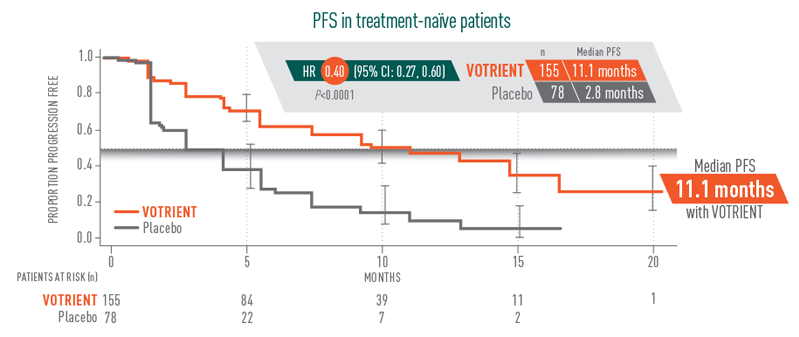
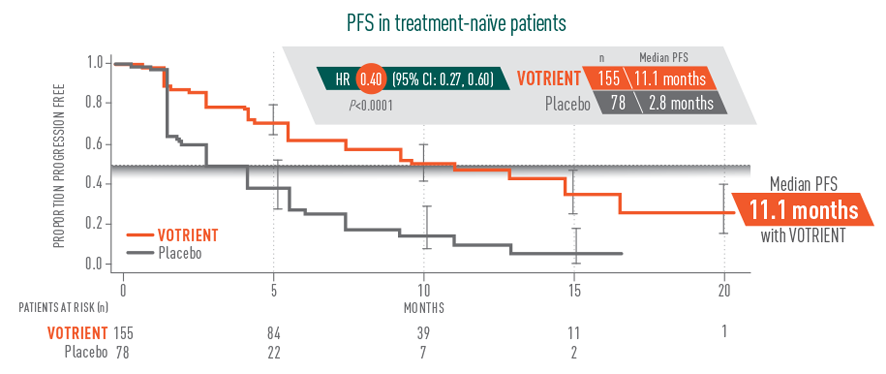
11.1 months median PFS for VOTRIENT first-line1,2
- 11.1 months median PFS achieved with VOTRIENT first-line vs 2.8 months with placebo in treatment-naïve patients
Patients responded to treatment in less than 2 months2
- For patients who responded to treatment with VOTRIENT, the median time to response was 11.9 weeks
*In patients pretreated with cytokines, VOTRIENT demonstrated a median PFS of 7.4 months vs 4.2 months for placebo (HR=0.54, P<0.001).1
References: 1. Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061-1068. 2. VOTRIENT [summary of product characteristics]. Camberley, UK: Novartis Europharm Limited; February 2018.