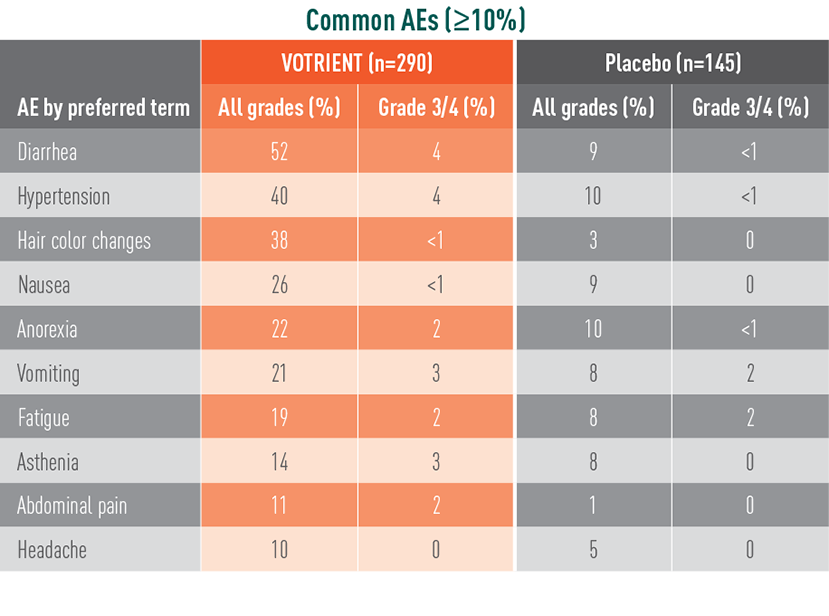
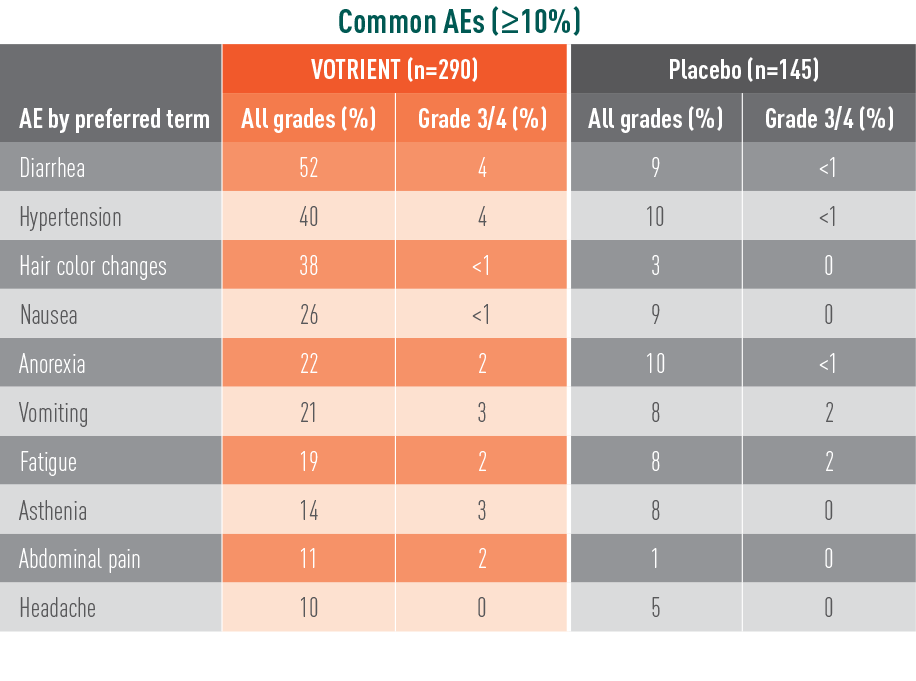
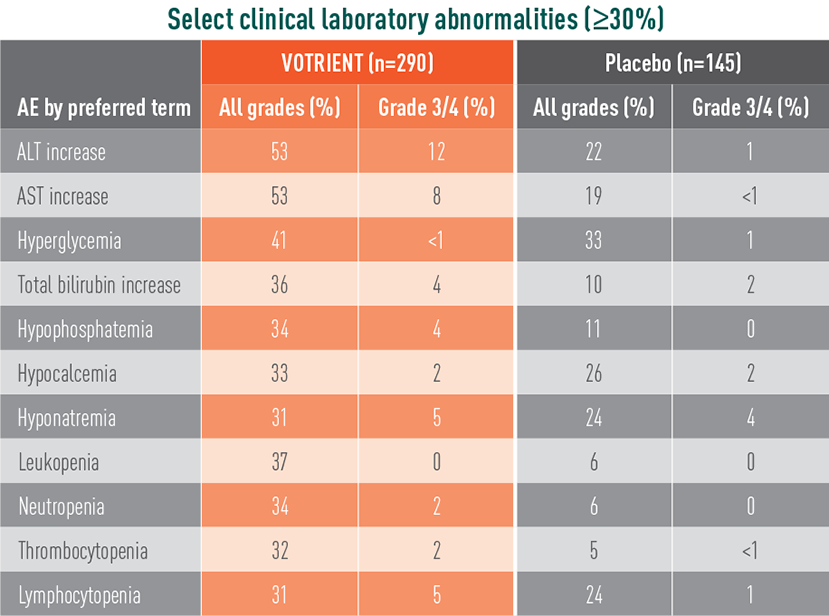
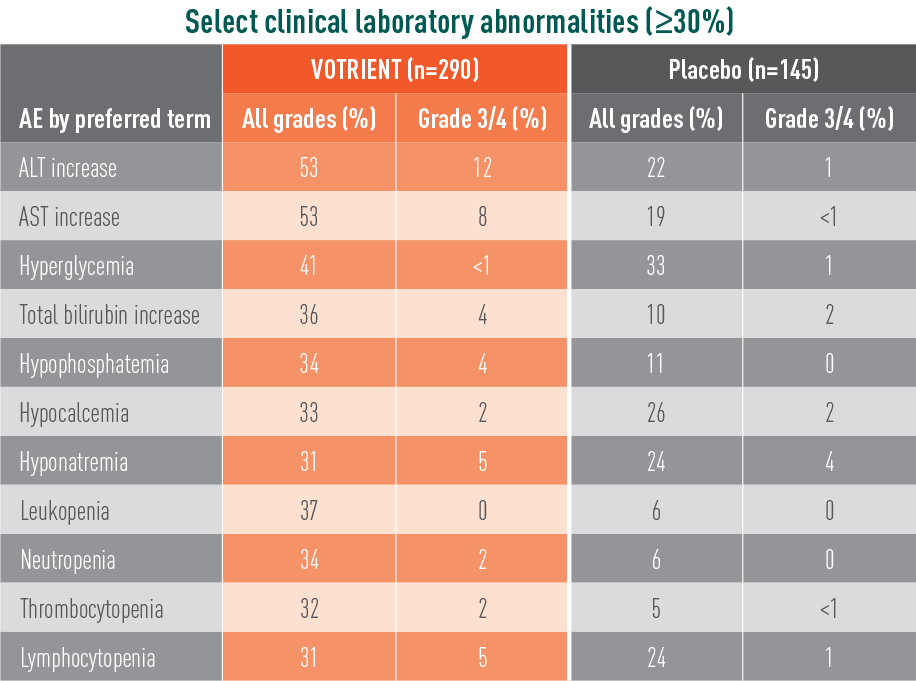
VOTRIENT—Most common adverse events (AEs) and laboratory abnormalities in the pivotal trial1
Most common AEs in the pivotal trial
- The most serious AEs for VOTRIENT in <1% of patients across all trials were transient ischemic attack, ischemic stroke, myocardial ischemia, myocardial and cerebral infarction, cardiac dysfunction, gastrointestinal perforation and fistula, QT prolongation, Torsade de Pointes, and pulmonary, gastrointestinal, and cerebral hemorrhage2
Most common laboratory abnormalities in the pivotal trial
- The most common (>5%) grade 3/4 laboratory abnormalities in the phase 3 trial were liver enzyme (ALT and AST) elevations
References: 1. Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061-1068. 2. VOTRIENT [summary of product characteristics]. Camberley, UK: Novartis Europharm Limited; February 2018.