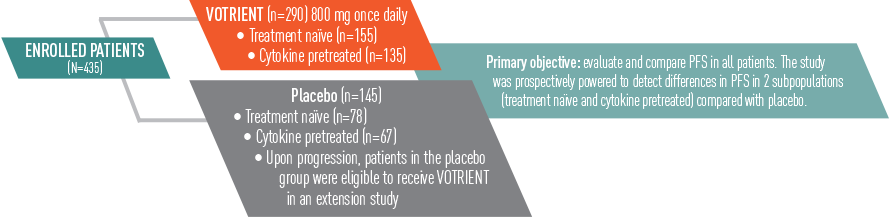
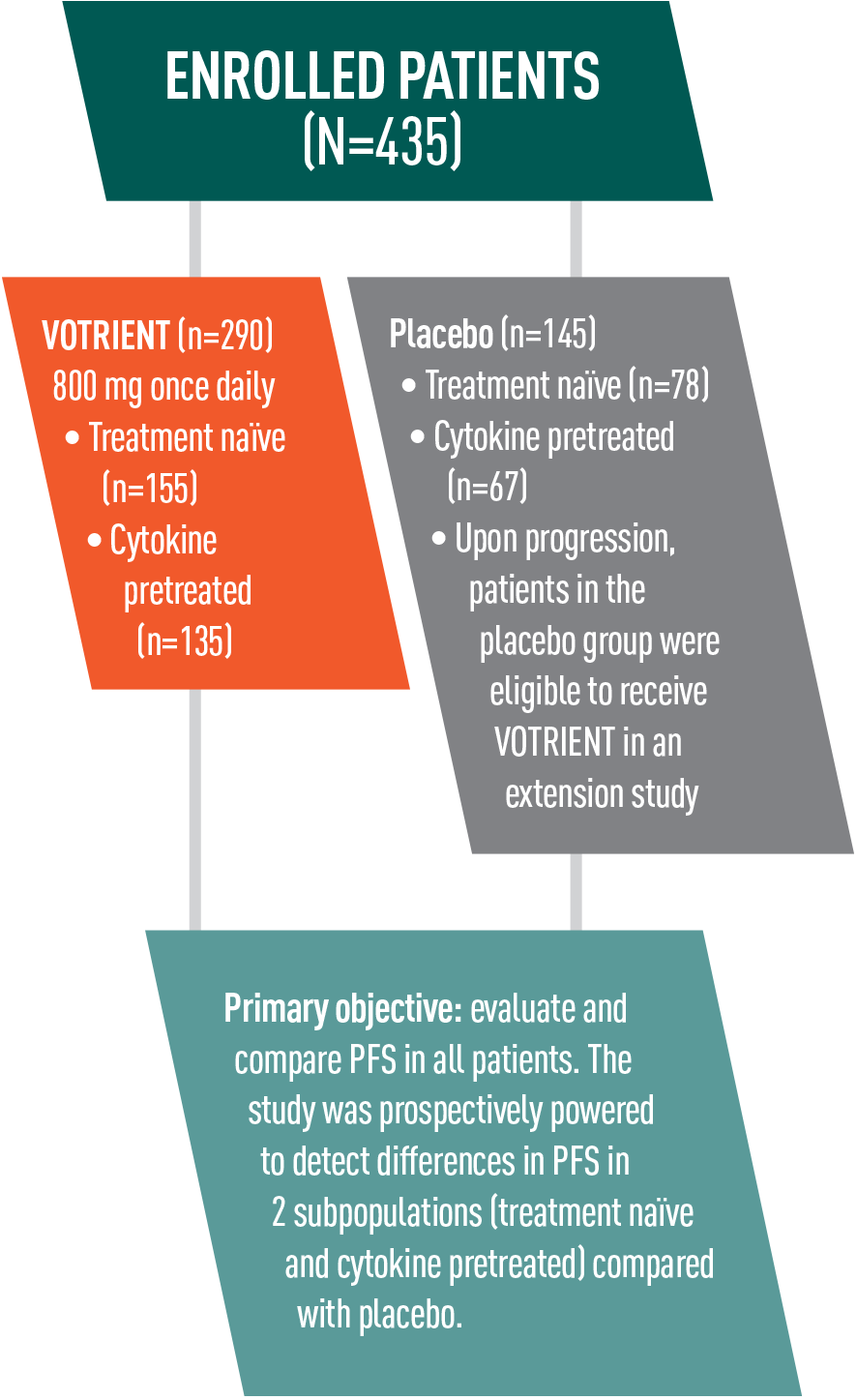
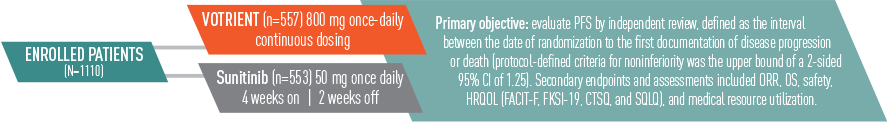
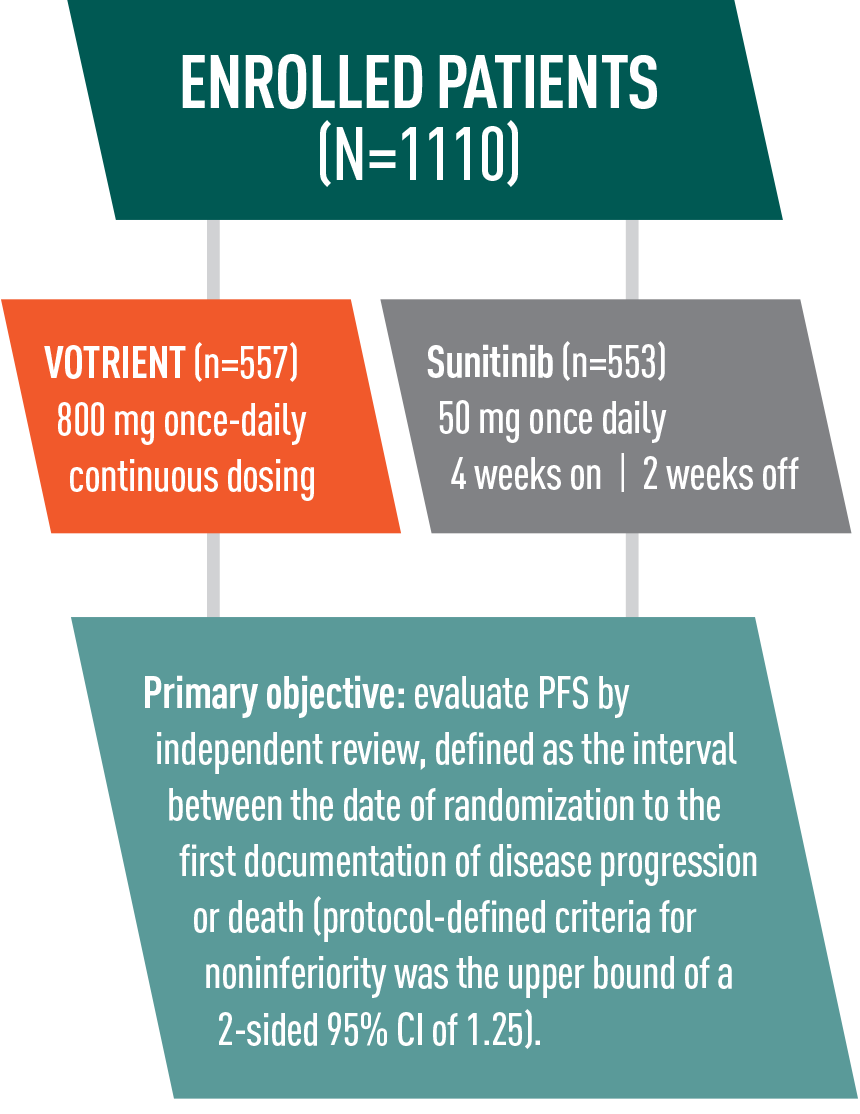
VOTRIENT—Proven in COMPARZ, the largest (N=1110) head-to-head trial in advanced renal cell carcinoma (RCC) vs sunitinib1-3
Comparable efficacy vs sunitinib by independent review
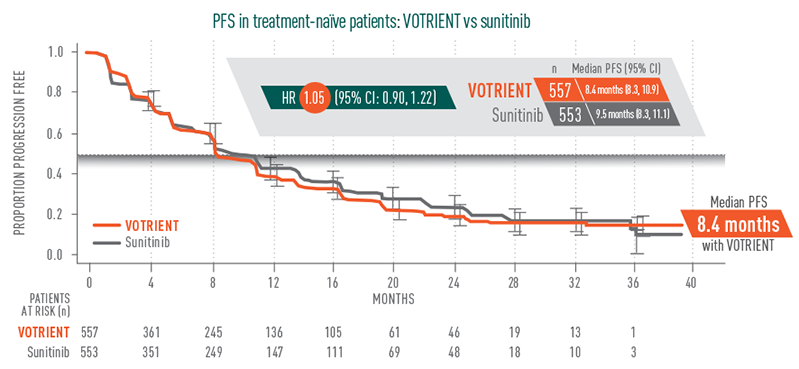
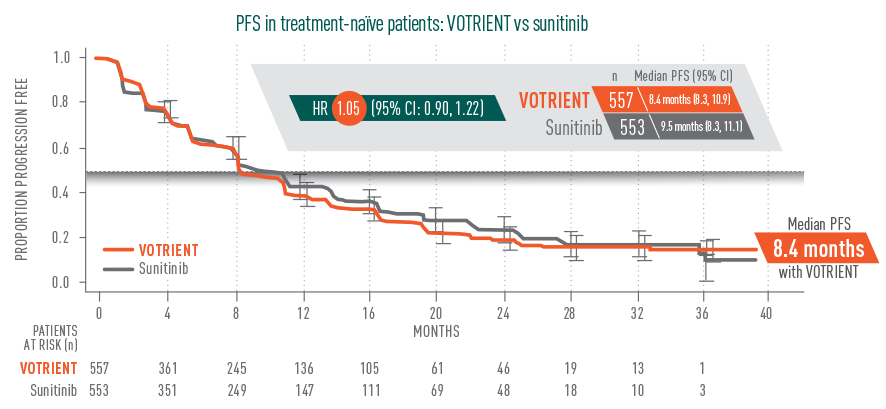
Progression-free survival (PFS): noninferior1*
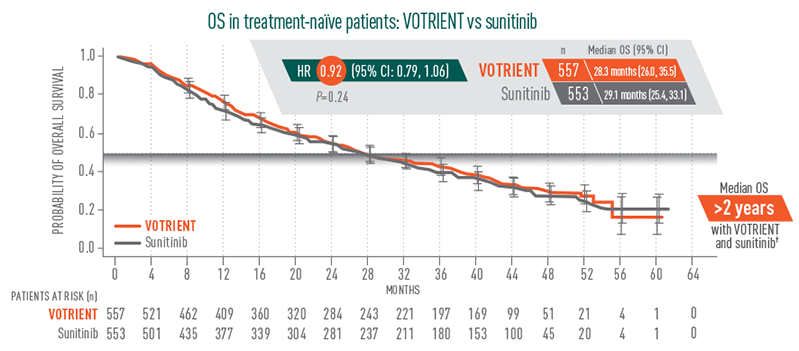
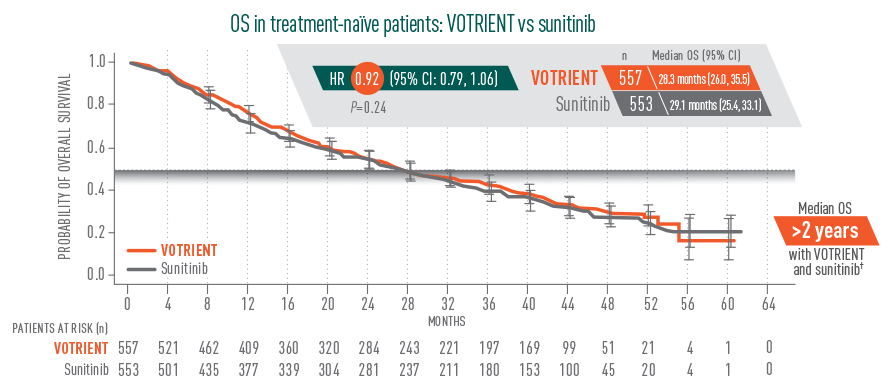
Overall survival (OS): >2 years2
No significant difference in median OS with VOTRIENT vs sunitinib
- In the pivotal trial, median OS for VOTRIENT was 22.9 months vs 20.5 months for placebo in the overall treatment population (HR=0.91; P=0.224)3
- There was no significant difference in OS for VOTRIENT vs placebo. Results were subject to potential bias because 54% of patients receiving placebo also received VOTRIENT in the study extension following disease progression
 In the treatment of advanced RCC, YOU HAVE VOTRIENT: A FIRST-LINE FORCE
In the treatment of advanced RCC, YOU HAVE VOTRIENT: A FIRST-LINE FORCE
Who is an appropriate VOTRIENT patient?
*The noninferiority margin was predefined as the upper bound of the 95% CI <1.25.
References: 1. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722-731. 2. Motzer RJ, Hutson TE, McCann L, Deen K, Choueiri TK. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib [letter to the editor]. N Engl J Med. 2014;370(18):1769-1770. 3. VOTRIENT [summary of product characteristics]. Camberley, UK: Novartis Europharm Limited; February 2018.